Samples and data withdrawal procedure
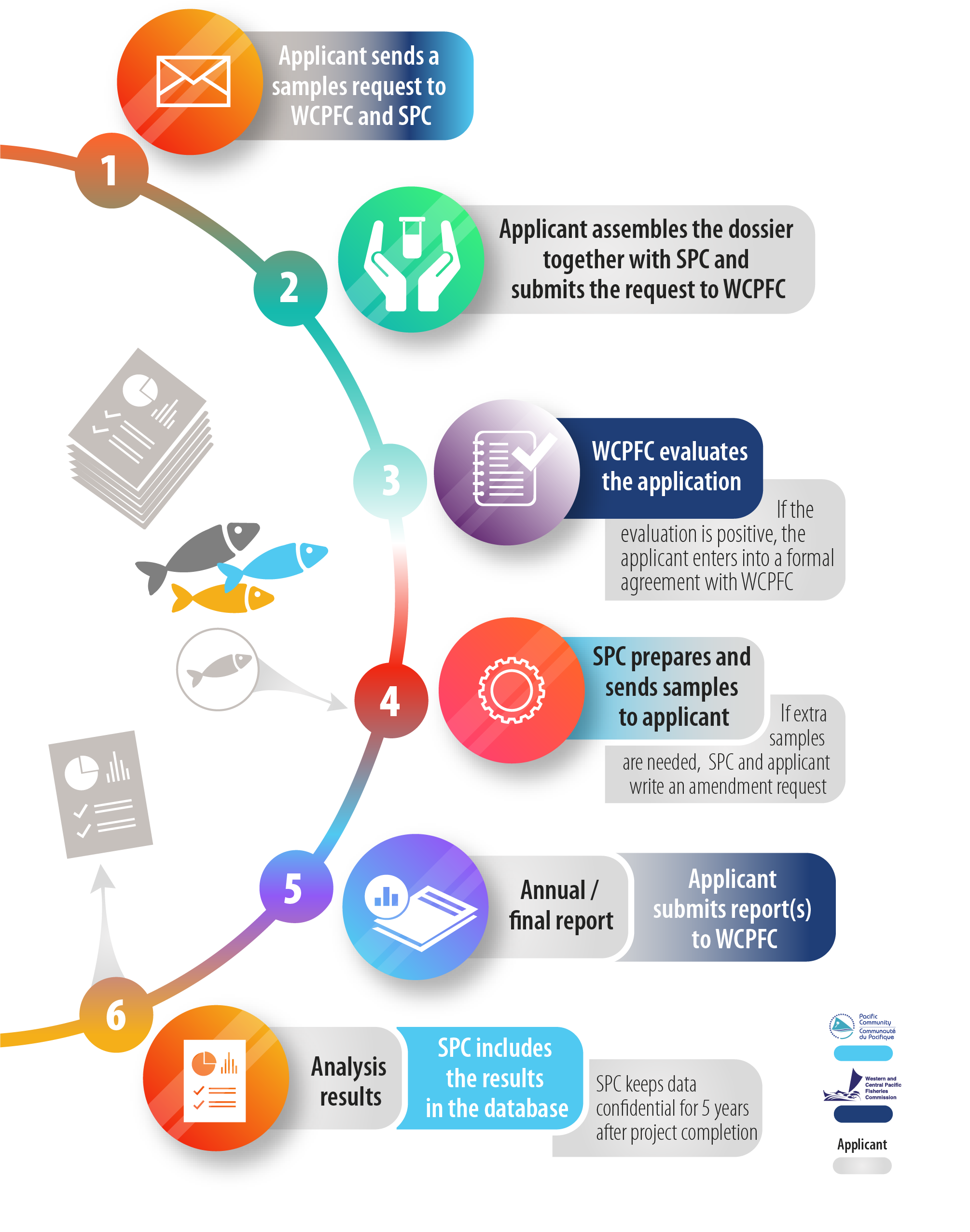
Step 1:
Please fill this form and send it to [email protected] and [email protected]
Step 2:
While the Search interface of this website gives you a good overview of the Tissue Bank resources, we can provide further information and advice (e.g. the latest updates on sample availability, ensure that your sample selection meets your scientific needs, customize sample selection for specific applications, etc.)
Applications to access samples from the Tissue Bank should be addressed to the Executive Director, WCPFC Secretariat, and must include:
- Project name and objectives
- WCPFC Scientific Committee Project Number or recommendation if these exist
- Specification of the samples to be withdrawn from the Tissue Bank (number, type, species, any location/sex/date limits, etc.)
- The methods for processing and analyses
- Past contributions to the Tissue Bank by researcher or CCM
- Intended collaborations
- Timelines and intended outcomes and reporting
Step 3:
The selection and approval of projects will be determined by the WCPFC Research Sub-Committee. This committee may meet within the margins of WCPFC meetings or electronically. This sub-committee will prepare and submit a summary of their decision on each project proposal to the WCFPC Executive Director for final approval. The project approval process will consider, inter alia, the following:
- Preferential access to the Tissue Bank will be given to researchers or WCPFC CCMs who have contributed samples to the collection.
- Preferential access to the Tissue Bank will be given to collaborative projects with priority to those where the collaboration includes several WCPFC CCMs.
- Priority will be given to requests that are part of the WCPFC Scientific Committee’s research and work plan and those projects whose spatial scale is regional in preference to local.
- Past participation, with those who acknowledge the source of the samples and provide interim products as required above given priority.
Once approval for access to samples from the Tissue Bank has been provided by the WCPFC Research Sub-Committee, the researcher/CCM will enter into a formal agreement with the Secretariat of the WCPFC that will specify access requirements, reporting and any data confidentiality that the WCPFC may require.
Step 4:
A reasonable fee may be charged for the cost associated with preparing the samples for shipping and cost recovery for freight or transport agent fees and freight (loss and damage) insurance. An additional fee will be charged to applications from organizations who are not associated with WCPFC CCMs. This fee will be based on the full cost recovery of the collection of samples requested. The total amount of this second fee that is collected in each year will be used to offset WCPFC’s costs of running the Tissue Bank in the following year.
Step 5:
It will be a requirement of the researcher or CCM to provide an annual report to the Executive Director, WCPFC Secretariat. This must include documentation of raw and analyzed results; however, this does not imply a requirement for this data to be made publicly available. When data can be made publicly available, a report to WCPFC’s Scientific Committee is required on the progress of the study. The reports must follow WCPFC standards and must include method descriptions and metadata.
Step 6:
Researchers or CCMs must acknowledge the WCPFC Tissue Bank in any publication of results from the study undertaken. Where the analyses involve the preparation of secondary products such as sectioned otoliths and histological slides, these products are to be provided to the WCPFC at the completion of the study for future comparative reference and study. All data will become publicly available 5 years after the WCPFC Secretariat determines the project analyses are complete or at WCPFC’s discretion.
 Pacific Marine Specimen Tissue Bank
Pacific Marine Specimen Tissue Bank